Acrolinx and RWS: Reducing the time to create, review, translate, and publish content
RWS is the world’s leading provider of technology-enabled language, content management, and intellectual property services. RWS Tridion Docs is an enterprise-wide Component Content Management System (CCMS) that helps customers author and collaborate on their content. It turns their content into intelligent information by providing granular content management capabilities, based on out-of-the-box support for the DITA standard, combined with Semantic AI capabilities. Tridion Docs is trusted by the world’s largest enterprises.

Add Acrolinx technology into the mix and, together, we can further improve the creation of in-depth content. We do so by providing clear and concise instructions at scale, centralizing processes, making sure quality controls are met, and introducing cross-product intelligence for better business outcomes.
The need to reduce ambiguities and complexity
For Knowledge Managers looking to reduce ambiguity and complexity within content, the key challenge is the same. How can you find a scalable way to create, review, translate, and publish your content?
The way forward is to centralize how content is created, managed, and maintained across different teams, languages, and contributors. Done well, this will improve both employee and customer experience by reducing editorial bottlenecks, quality issues, and compliance risks.
Achieve greater efficiency and better compliance
Working in tandem, and by centralizing processes, RWS and Acrolinx can ensure quality controls are met. We also improve product development through code optimization and consistent terminology.
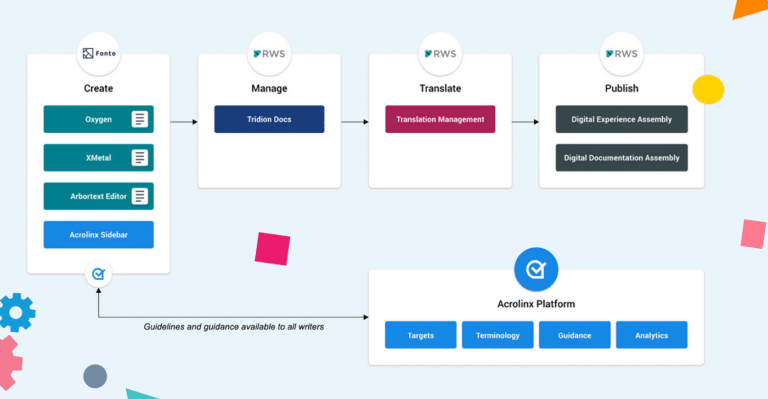
The diagram below shows how seamlessly Acrolinx plugs into the RWS process to provide a complete platform for structured content authoring, management, and governance.
Improving in-depth information across many industries
In partnership with RWS, we can build and improve the creation of in-depth technical and product information across many industry sectors. These include Hi Tech, Software, Appliances, Automotive, Heavy Manufacturing, and Medical. Whatever the industry, we can deliver an integrated content management and governance solution, including language translation and localization.
This solution enables easy collaboration between subject matter experts (SMEs) and technical authors who can address a wide range of knowledge and in-depth information situations. Its use cases span technical product information, service and support information, policies, procedures, and any other type of structured, highly controlled content.
At the same time, it allows you to easily reuse, share, filter, and deliver multilingual content to any channel. It drives improved productivity and information findability, helps you avoid content inconsistencies, and can reduce content development costs by up to 50%.
Where to go for more information
If you’d like to learn more about Acrolinx’s partnership with RWS, please check out the following assets:
In partnership with RWS, we can build and improve the creation of technical content, by providing clear and concise instructions at scale, centralizing processes, making sure quality controls are met, and introducing cross-product intelligence for better business outcomes.
The Med Tech indusrty is growing fast — and so is the need for content to support its products. Learn how RWS and Acrolinx can help.
Disparate digital experiences (DX) are one of the biggest challenges businesses face today. Poorly thought out or siloed content can not only prevent enterprises from creating the best …
Acrolinx and Fonto Align Writers to Create Impactful Content
See the power of Acrolinx and Fonto, part of the RWS Group, working together to help make the writing process easier and more accurate, while optimizing it for content reuse.
For more information on our partner program, contact Dan Nutburn, Vice President, Global Partners and Alliances.
Ready for content success?
See how our AI capabilities help you create and maintain high-quality content in our demo.