To introduce a new drug to the market, it needs to undergo an approval process. This process also covers content. And we’re speaking about a lot of content pharma companies have to produce:
- Investigational New Drug (IND) Application
- Chemistry, Manufacturing, and Controls (CMC) Information
- Clinical Trial Protocols
- Clinical Study Reports (CSRs)
- Nonclinical Study Reports
- Integrated Summary of Safety (ISS) and Integrated Summary of Efficacy (ISE)
- Risk Evaluation and Mitigation Strategy (REMS) Plan
- Labeling
- Pharmacovigilance Plan
- Marketing Materials
The good thing is: The FDA offers content guidance. “Guidance documents […] do not create or confer any rights for or on any person and do not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations.”
But they provide pharma companies a framework for successful approval of new drugs or medical devices.
How to structure your pharmaceutical content
Content clarity is essential
Always keep in mind that some of the documents you write target non-experts. Your target audience is extremely diverse. Make sure to talk to everybody with your content. Thus, you need to make sure you’re as clear as possible, without making any compromises on scientific correctness. Clearer content reduces the need for FDA reviewers to send clarification requests or worse, misinterpret information.
To write clearer content, it’s important to follow clarity style guidelines like using short sentences, but also by aspects like scannability or terminological consistency.
Structure and scannability
Here are some exemplary aspects touching upon content, cited from “Guidance for Industry Labeling OTC Human Drug Products”, “V. CONTENT REQUIREMENTS (21 CFR 201.66(c))5” section. This section defines content components and order, and also touches on content structure and style.
- Use bulleted lists to “make the presentation of the information clearer and easier to read.” (p. 7)
- “Listing too many alternative ingredients could be misleading and could cause consumer confusion. To avoid such confusion, manufacturers, packers, and distributors may wish to consider using a second set of labels for drug products with a lengthy list of different inactive ingredients.” (p. 13 )
Using compliant terminology and sentence structure
You have to make sure you use the correct medical terminology and binding phrases or sentences.
Correctness is mandatory
In addition to other things to consider, make sure your copy is absolutely error-free. A trivial typo is enough to mess up the approval process by causing additional review rounds.
Being responsible for those language-related aspects is basically a full-time job. Assuming that you work in the Product or R&D department: Is there a way to expedite FDA approval without content compliance headaches?
One common approach to at least minimize content compliance headaches is reusing “pre-approved content modules” wherever applicable. There are multiple benefits, for instance, more content is approved in only one review cycle, which influences time to market and costs positively (source).
You can input Acrolinx your approved messaging – it reminds you to use it while you create content. That alone boosts both efficiency and compliance, but our platform also helps:
- Improving clarity, scannability and other aspects of language that resonates with your audience
- Making sure you use the correct medical terminology
That might sound like a lot, but it’s no rocket science. Let’s discover what Acrolinx exactly does to make work in the pharma industry easier.
How Acrolinx helps you create compliant content
Step 1: Integrate Acrolinx into your content workflow
Before the magic happens, feed Acrolinx by providing it everything it needs to consider. First, identify the following and configure them in your Acrolinx instance:
- industry terms
- unit measurement representations
- Acronyms
- Proper spelling and representation of:
- Symptoms
- Side effects
- Elements
- Chemical structure
Then, define any terminology that shouldn’t be used. Once defined, Acrolinx alerts writers if it finds a non-compliant term and provides a prefe
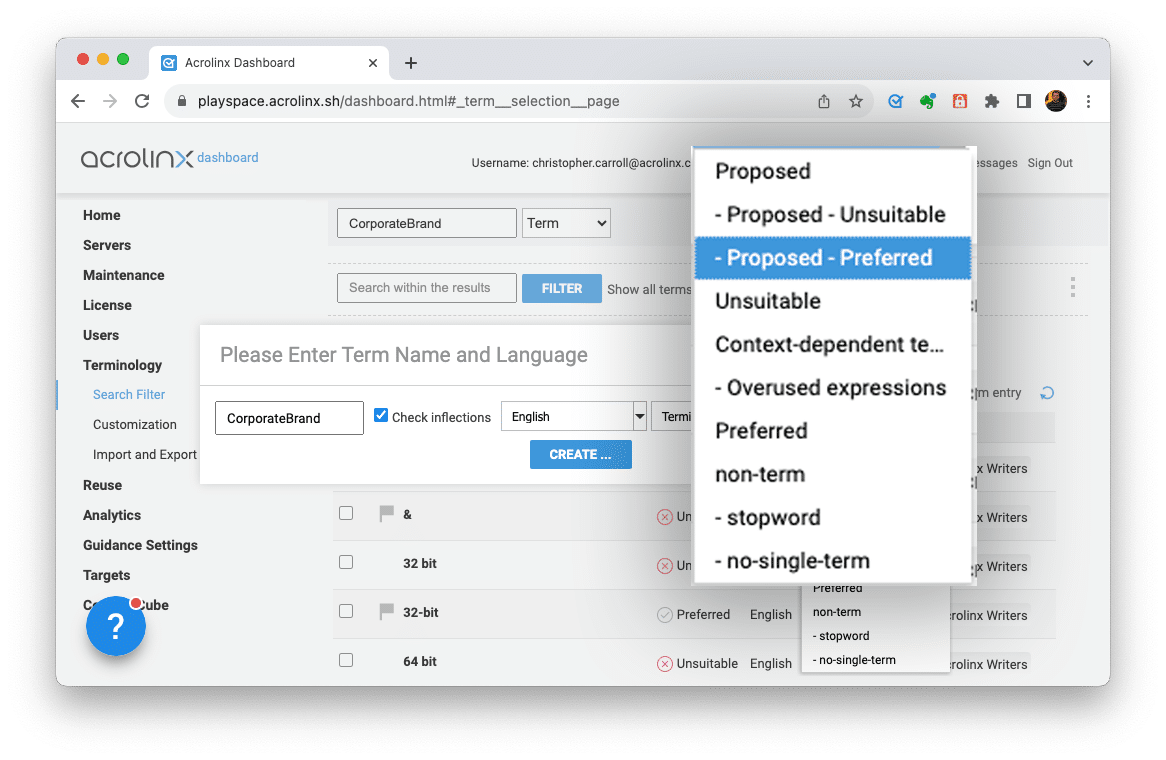
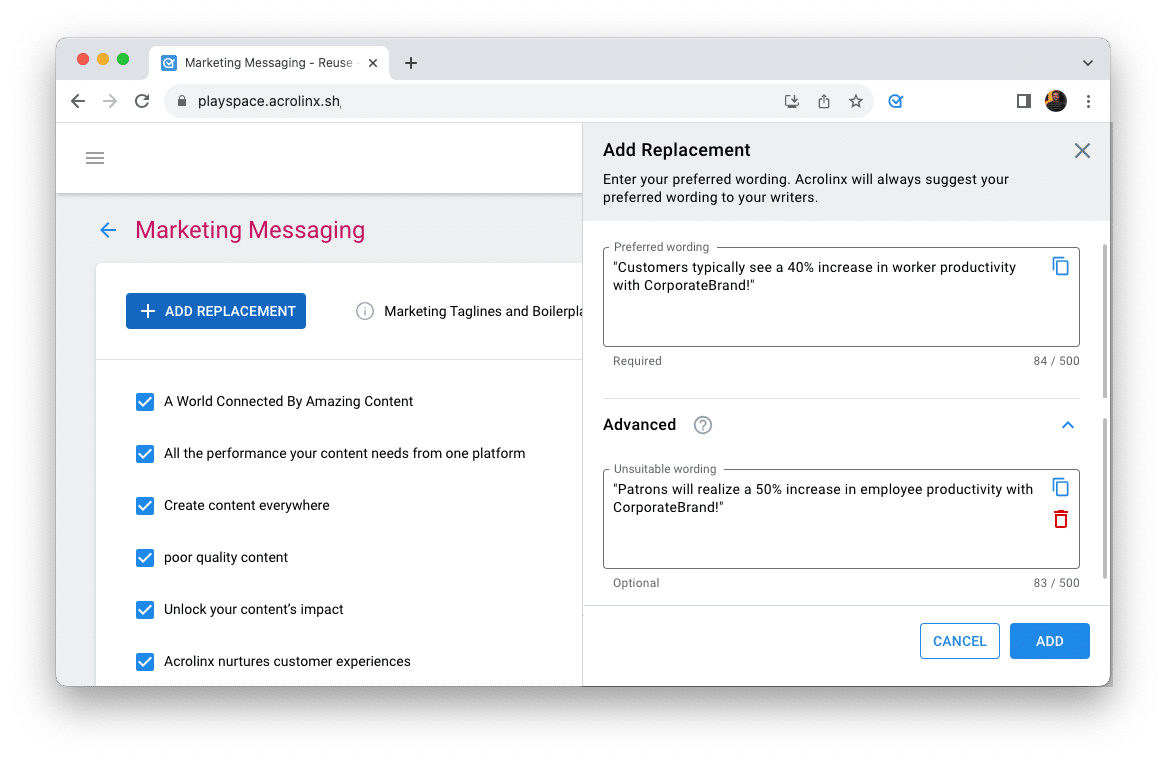
Step 2: Implement Approved Messaging for compliance
Then we’re leaving the word level. Identify disclaimers, disclosures, statements, and paragraphs that must be used verbatim because of regulatory requirements.
In the Acrolinx Dashboard, you can add this Approved Messaging. When writers create similar but different content, Acrolinx offers the approved disclaimers, statements, or paragraphs to be inserted on-click.
Step 3: Set structure and consistency guidelines
Well-structured, consistent content helps both consumers and FDA reviewers with improved clarity and predictability. Set guidelines that you’re using later to configure Acrolinx. These guidelines may contain the following:
- Proper capitalization
- Unit measurement and other labeling
- Sentence, paragraph, and list lengths
- Headings, content, and structural formatting
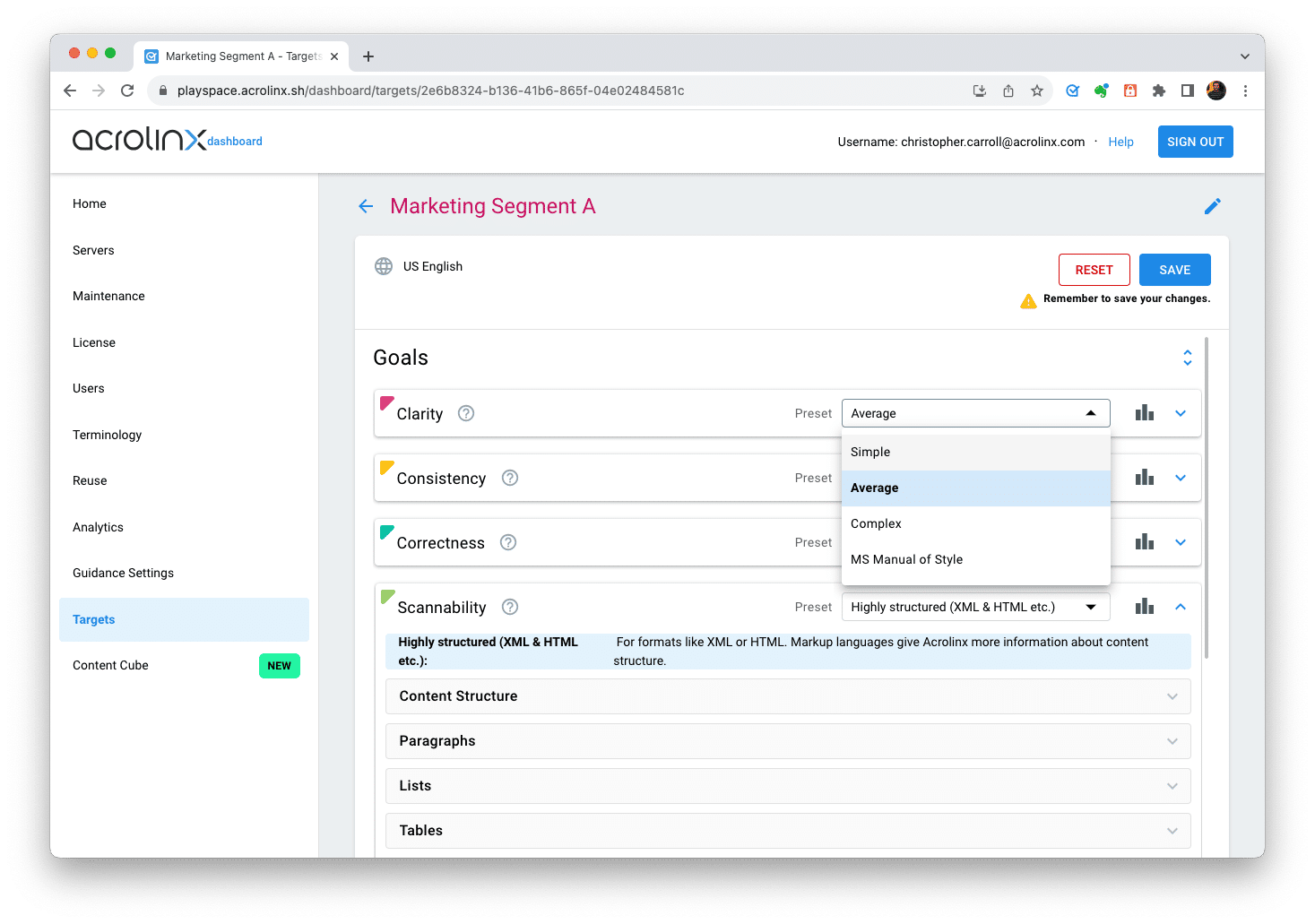
Step 4: Create an audience target
Now it’s time to combine all parts into an audience target. In the Acrolinx Dashboard, create an audience target and do the following:
- Set ideal clarity, scannability, and consistency, see step 3.
- Connect your target to the terminology, acronyms, and Approved Messaging you configured in step 1 and 2.
Step 5: Scan your content for compliance issues
Now it’s time to make sure your content is compliant with the goals you set before – and thus with the FDA’s guidelines and legal regulations. Acrolinx lets you:
- Scan content for quality issues and focus on terminology or approved messaging for compliance issues.
- Measure reading ease scores to understand expected comprehension reading levels.
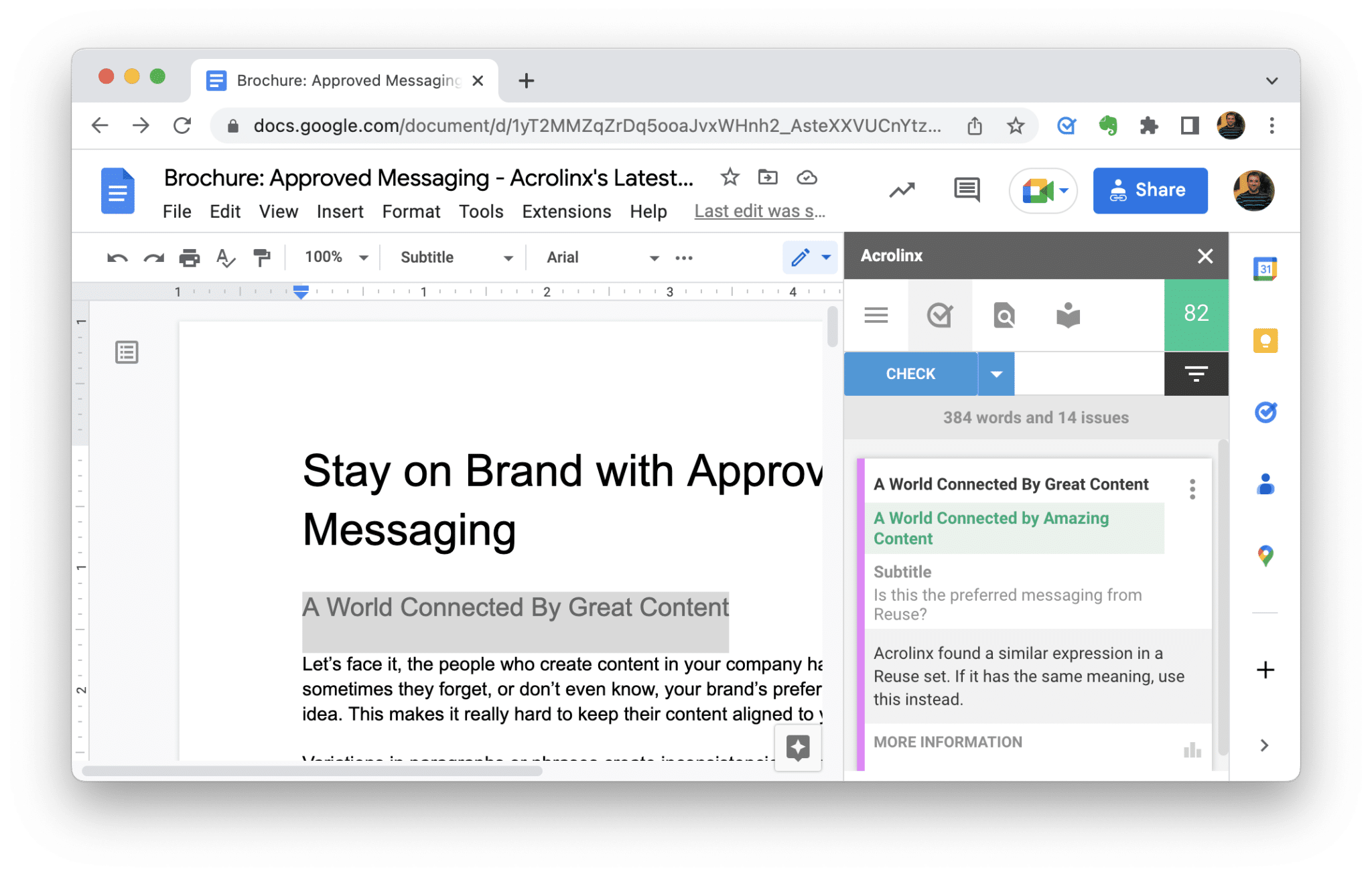
- View detail types of content issues, where they are in content and recommended fixes in Acrolinx Scorecards.
If Acrolinx is used during content creation, the Acrolinx Sidebar gives writers timely feedback on compliance issues related to improper terminology or inappropriately written disclosures.
Step 6: Share content issues with writers based on severity
Content editing is crucial for content that is mandatory for FDA approval. So this step is all about checking your writer’s output, making sure to detect remaining content issues. There’s 90% less content issues if Acrolinx is used during content creation, but in case your editors find something nevertheless, it’s their turn to approach writers and share the content issues with them, based on severity.

Step 7: Writers revise and publish FDA-compliant content
With the feedback they got from their content editors, the final step is for the writers to update and publish the corrected content. The result is improved overall clarity, less brand issues, and a higher Acrolinx score that stands for great content that’s likely to pass FDA review.
Fast-track your FDA approval with Acrolinx
Achieving pharma content approval and securing FDA approval doesn’t have to be a slow, complex process. Pharma companies can streamline documentation and reduce regulatory delays by implementing structured content strategies, ensuring pharma content compliance, and leveraging advanced tools like Acrolinx.
Focusing on clarity, consistency, and correctness in your content ensures it meets FDA standards while minimizing revision cycles. To get your product FDA-approved faster, you need to optimize your content strategically. Your brand credibility will increase and life-saving products will reach the market faster if you prioritize regulatory alignment, content automation, and proactive compliance measures.
So, what are you waiting for? Connect with us and discover Acrolinx as your solution to expedite FDA approval.
Frequently asked questions
Adherence to internal standards and external regulations. Acrolinx enforces compliance across content types automatically.
It includes patient-facing materials, clinical documentation, and labeling. Acrolinx helps ensure this content is accurate, compliant, and clear.
It varies — months to years depending on the product. Acrolinx supports some aspects of content readiness to support faster approvals.
They manage content workflows. Paired with Acrolinx, teams can ensure regulatory clarity and consistency from draft to submission.
Approval requires clinical trials, while clearance is for lower-risk devices. Acrolinx helps making sure all supporting content is compliant and well-documented.
Are you ready to create more content faster?
Schedule a demo to see how content governance and AI guardrails will drastically improve content quality, compliance, and efficiency.

Hannah Kaufhold
is a Content Strategist and Global Product Marketing Manager at Acrolinx, with over ten years experience in content strategy and content creation. They hold a Master’s degree in linguistics. Hannah has a strong interest in controlled languages and terminology and is passionate about diversity and inclusion. In their free time, they enjoy spending time with their family and reading.